Overview
AniTech Limited, a pre-clinical contract research organization (CRO), offers a full range of animal and cell-based testing services. This integrated approach ensures compliance with regulatory requirements set by government agencies such as the Food and Drug Administration (FDA) in the US, National Medical Products Administration (NMPA) in China and European Medicines Agency (EMA) in the European Union. We offer comprehensive testing services and regulatory filing documentation support for the efficacy and safety assessment of various products, including pharmaceuticals, medical devices, agricultural products, cosmetics, and supplementary health products. The company’s expertise and services extend to multiple industries, ensuring that clients can meet the necessary regulatory requirements. Furthermore, AniTech’s customizable pre-clinical studies services cater to the specific needs and requirements of clients, allowing for tailored testing protocols. This flexibility enables efficient and targeted evaluation of drug candidates while minimizing unnecessary costs.
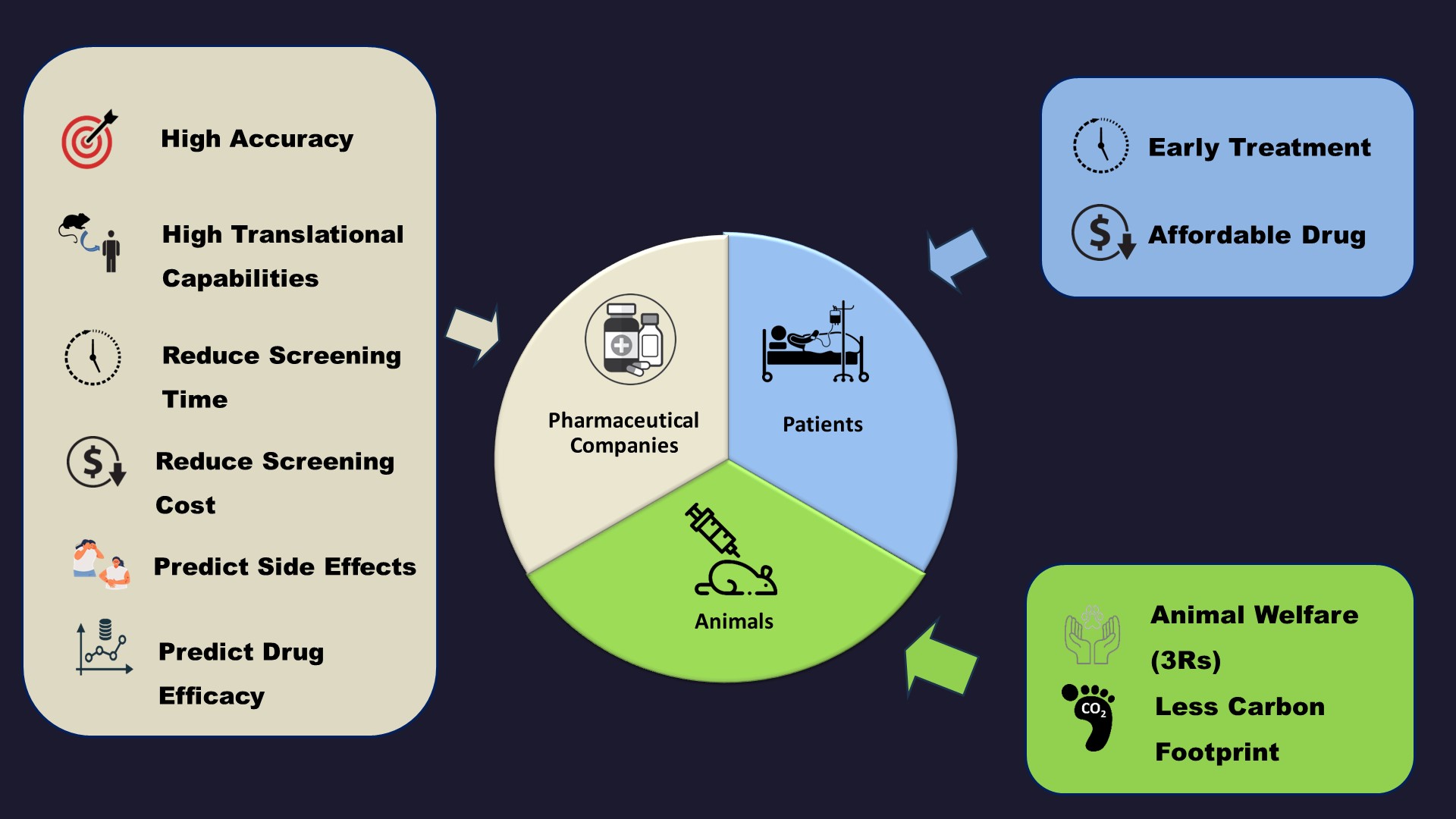
Application of Our Technology
⋆ Novel Drug Screening
⋆ Safety and efficacy evaluation of novel or repurposed drugs
⋆ Safety screening for cosmetic and health supplement products
⋆ Safety evaluation for medical devices
⋆ Safety evaluation for food product and food packaging
Our Intellectual Property

Patent ID: 17/883,619
Awards
Local and International Recognitions

Gold Medal
City University of Hong Kong

Silver Medal
Innovation and Technology Commission

Science Pioneer Award
YLOT
Funding Support
We are proudly supported by these institutions

HKTech300
City University of Hong Kong

TSSSU
Innovation and Technology Commission

CCMF
Cyberport Hong Kong
